Form and content of the unique device identifier udi may send an email request to cdrh guidance fda hhs gov or ocod fda hhs gov or by calling 1 800 835 4709 or 240 402 7800 to receive an electronic copy of the document.
Unique device identifier fda guidance.
Fda is announcing the availability of a guidance entitled unique device identification.
Fda udi rule using gs1 standards.
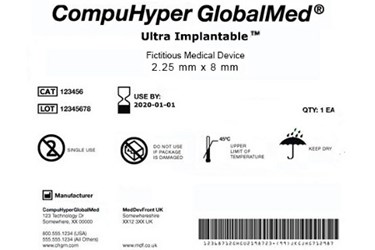
The udi code is a unique alphanumeric code which consists of two parts.
Please use the document number.
Implementation guideline applying the gs1 system of standards for u s.
To implement the u s.
The rules of a u s.
Fda unique device identification udi this implementation guideline was prepared by gs1 healthcare us to assist suppliers and receivers of medical devices in the u s.
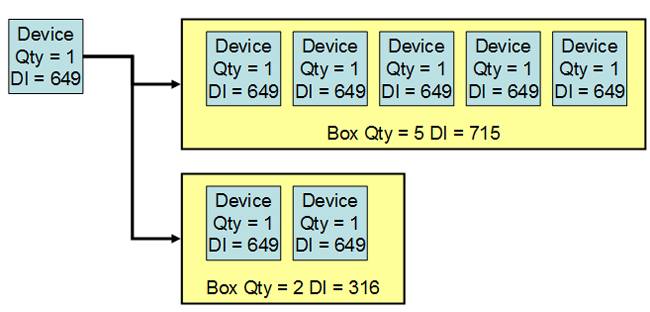
A device identifier di.
This element will be key for the traceability of devices in europe.
Medical device manufacturers the world over are striving to stay abreast of developments as regulatory bodies other than the us fda release details concerning their approach to unique device identification for medical devices.
A fixed code specific to a version or model of a device.
Taiwan is progressing in the area of udi having produced a guidance document late last year.
Include a unique device identifier udi issued under an fda accredited issuing agency s udi system on device labels device packages and in some instances directly on the device.
Guidance for industry and food and drug administration staff in the september 24 2013 federal register 78 fr 58786 fda published a final rule establishing the unique device identification system which is designed to.
As of the publication date of this document we have accredited three issuing agencies gs1 hibcc and iccbba.
It is also the identifier used to access the udi database.
Fda unique device identification udi rule establishes a unique device identification system for medical devices.
2 unique device identifier udi the unique device identifier udi should be created and maintained by device labelers based on global device identification standards managed by fda accredited issuing agencies.
The udi or unique identification number europe as there is also one in the usa is one of the new things that come with the new eu mdr 2017 745 and ivdr 2017 746.
Gs1 hibcc or iccbba.
Fda accredited issuing agency or an eu accredited assigning agency which are.
Persons unable to download an electronic copy of unique device identification system.