All reusable devices subject to dm udi on the device itself dm udi may be provided through either or both.
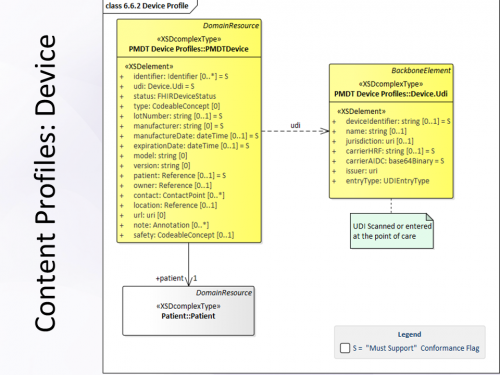
Udi for reusable medical devices.
Udi labelling will be required for class i devices from 26thmay 2025.
A class ii device that is required to be labeled with a udi must bear a udi as a permanent marking on the device itself if the device is a device.
The udi or unique identification number europe as there is also one in the usa is one of the new things that come with the new eu mdr 2017 745 and ivdr 2017 746.
For additional information on udi see the imdrf udi guidance document of december 2013.
Medical devices and performance study investigational devices.
This element will be key for the traceability of devices in europe.
Under 21 cfr 801 45 a device that must bear a unique device identifier udi on its label must also bear a permanent marking providing the udi on the device itself if the device is intended to.
The udi system is intended to provide a single globally harmonized system for positive identification of medical devices.
The udi carrier shall be placed on the label of the device and on all higher levels of packaging and in case of reusable devices on the device itself direct marking.
Udi and gs1 gs1 is an udi issuing agency entity based on many regulations worldwide in particular us eu china south korea saudi arabia meaning that manufacturers supplying regulated medical devices to these markets can use the gs1 standards to implement the udi requirements.
In the case of reusable devices the udi is additionally required on the device itself but only two years after the date of application on the labelling for the respective class of device.